Abstract
Introduction
Infant acute leukemia commonly involves high-risk cytogenetic abnormalities, with a significant portion those affected suffering early relapse and death. Allogeneic hematopoietic cell transplantation (HCT) is often pursued with curative intent and umbilical cord blood (UCB) grafts provide a readily available stem cell source. Myeloablative conditioning (MAC) is the standard of care for these patients, however total body irradiation (TBI)-based regimens raise concern for subsequent neurodevelopmental delays. The multi-institutional COBLT study evaluated UCB transplant (UCBT) following busulfan/melphalan/equine anti-thymocyte globulin MAC for infant leukemia and found an incidence of day 42 neutrophil recovery of 0.59 (95% confidence interval [CI], 0.44-0.78) and 2-year relapse-free survival of 0.28 (95% CI: 0.13, 0.44; Wall DA et al., BBMT 2005). We hypothesized that replacing anti-thymocyte globulin with fludarabine and increasing the dose of melphalan would improve engraftment and relapse-free survival in UCBT for infant hematologic malignancies.
Methods
In a phase II study of UCBT for infant hematologic malignancies, we assessed routine HCT outcomes following myeloablative conditioning delivered to 2 consecutive cohorts: Cohort A: Busulfan (IV, target steady state 600-900 ng/ml over 4 days), melphalan 180 mg/m2 and fludarabine 75 mg/m2 (November 2005 - March 2015); and Cohort B: Busulfan (IV, target cumulative exposure 14,080-16,400 uM*min/L over 4 days), melphalan 150 mg/m2 and fludarabine 75 mg/m2 (or 2.5 mg/kg if <10kg and/or <1 year old) (April 2015-May 2021). Reduction in melphalan aimed to reduce regimen toxicity; change in busulfan area under the curve target from daily to cumulative measurement reflecting best practices, and transition from double UCB to mismatched UCB stem cell source aimed to increase the graft-versus-leukemia effect. Graft-versus-host disease (GvHD) prophylaxis included a calcineurin inhibitor (cyclosporine or tacrolimus) and mycophenolate mofetil. Eligibility included age ≤ 3 years at diagnosis, and acute leukemia in complete remission (high risk CR1 or subsequent CR) or myelodysplastic. The cumulative incidence of time-dependent events was compared between cohorts using the Gray test with death as a competing risk. Estimates of overall survival were calculated by Kaplan-Meier analysis.
Results
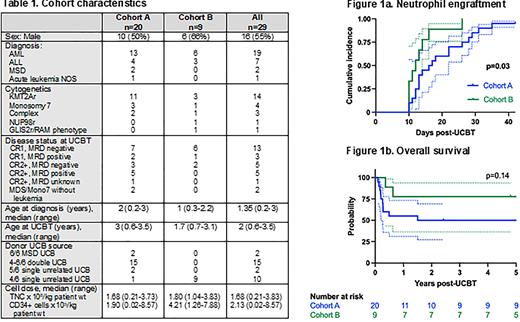
Twenty-nine patients underwent UCBT, 20 in cohort A with a median follow-up of 717 days (range 34-5821) and 9 in cohort B with a median follow up of 1859 days (range 131-2610). Demographics and transplant characteristics are shown in Table 1, notable for a greater proportion of Cohort A patients with minimal residual disease (MRD) positivity measured by flow cytometry at time of UCBT. Neutrophil engraftment was achieved at a median of 16 days for Cohort A and 12 days for Cohorts B (p=0.03; Fig 1a). One Cohort A patient had primary graft failure successfully salvaged by a 2nd UCBT. The cumulative incidence of Grade II-IV acute GvHD by 100 days post-UCBT was 0.45 (95% CI 0.22, 0.65) in Cohort A, 0.22 (95% CI 0.03, 0.53) in Cohort B, p=0.26. One Cohort A patient died of chronic GVHD, no other chronic GVHD was reported. 2-year cumulative incidence of relapse was 0.15 (95% CI 0.04, 0.34) in Cohort A, 0.33 (95% CI 0.07, 0.64) in Cohort B, p=0.28. Of note, 3 of the 4 relapses in Cohort B were successfully salvaged with CAR-T therapy and/or second HCT. 2-year non-relapse mortality (NRM) was 0.35 (95% CI 0.15, 0.56) in Cohort A and 0 in Cohort B as the 2 deaths in this cohort were following relapse, p=0.049. 2-year overall survival was 0.5 (95% CI 0.27, 0.69) in Cohort A and 0.78 (95% CI 0.36, 0.94) in Cohort B, with a hazard ratio of death for Cohort A compared to B of 2.44 (95% CI 0.75, 7.87; Fig 1b).
Discussion
Myeloablative HCT for infants with acute leukemia with a preparative regimen consisting of busulfan, fludarabine and melphalan and an UCB graft results in high levels of engraftment. Compared to the prior COBLT study in this patient population, rates of engraftment were significantly higher and relapse-free survival and overall survival were comparable, limited by small sample size in this study. Further improvement in outcomes could potentially be achieved by incorporating post-transplant relapse mitigating strategies as well as supportive care measures to decrease regimen-related toxicities.
Disclosures
Wagner:ASC Therapeutics: Consultancy; Bluebird Bio: Consultancy; Vertex Pharmaceuticals: Consultancy; Magenta Therapeutics: Consultancy; Rocket Pharmaceuticals, Inc.: Consultancy, Current equity holder in publicly-traded company. Verneris:Up-To-Date: Consultancy; Bmogen: Consultancy, Current equity holder in private company; Fate Therapeutics: Consultancy, Current equity holder in private company; Novartis: Membership on an entity's Board of Directors or advisory committees.
Author notes
∗Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal